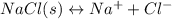Which statement best describes how dynamic equilibrium relates to a saturated solution of sodium chloride? the concentration of the dissolved particles remains constant. no more solid dissolves in a saturated solution. each second, the amount of solid sodium chloride that dissolves equals the amount of solid sodium chloride that recrystallizes. each second, solid sodium chloride dissolves and solid sodium chloride recrystallizes.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Which statement best describes how dynamic equilibrium relates to a saturated solution of sodium chl...
Questions

Biology, 12.06.2021 03:20



Social Studies, 12.06.2021 03:20

Mathematics, 12.06.2021 03:20


Mathematics, 12.06.2021 03:20



Mathematics, 12.06.2021 03:20





English, 12.06.2021 03:20



Mathematics, 12.06.2021 03:20

Biology, 12.06.2021 03:20

Chemistry, 12.06.2021 03:20