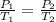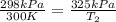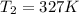Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
The pressure in a car tire is 298 kilopascals at 300 kelvin. after a long drive, the pressure become...
Questions







Chemistry, 28.08.2019 19:10





Health, 28.08.2019 19:10




Computers and Technology, 28.08.2019 19:10


Business, 28.08.2019 19:10


Biology, 28.08.2019 19:10

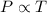 (At constant volume and number of moles)
(At constant volume and number of moles)