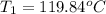Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 115 °c was dropped into a calorimeter containing 57 g of water (specific heat 4.184 j/g*°c). the final temperature of the glass and water in the calorimeter was 119.2 °c. what was the initial temperature of the water?
39.84°c
79.68°c
119.84°c
139.68°c

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 1...
Questions



Mathematics, 24.07.2019 11:30

Arts, 24.07.2019 11:30

Mathematics, 24.07.2019 11:30

Mathematics, 24.07.2019 11:30



Health, 24.07.2019 11:30



Mathematics, 24.07.2019 11:40

Mathematics, 24.07.2019 11:40



Mathematics, 24.07.2019 11:40



Mathematics, 24.07.2019 11:40

History, 24.07.2019 11:40

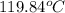
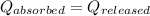
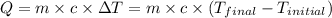
![m_1\times c_g\times (T_{final}-T_2)=-[m_2\times c_w\times (T_{final}-T_1)]](/tpl/images/0305/5772/96ec8.png) .................(1)
.................(1)
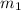 = mass of glass = 32.50 g
= mass of glass = 32.50 g
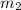 = mass of water = 57 g
= mass of water = 57 g
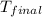 = final temperature of water and glass =
= final temperature of water and glass = 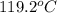
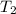 = initial temperature of water = ?
= initial temperature of water = ?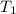 = initial temperature glass =
= initial temperature glass = 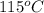
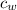 = specific heat of water =
= specific heat of water = 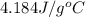
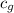 = specific heat of glass =
= specific heat of glass = 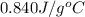
![(32.50g)\times (0.840J/g^oC)\times (119.2^oC-115^oC)=-[(57g)\times (4.184J/g^oC)\times (119.2^oC-T_1)]](/tpl/images/0305/5772/c0a00.png)