The electron configuration for magnesium is shown below.
1s22s22p63s2
which...

Chemistry, 13.10.2019 02:50 ayoismeisjuam
The electron configuration for magnesium is shown below.
1s22s22p63s2
which diagram shows the correct distribution of electrons in the electron shells of a magnesium atom?
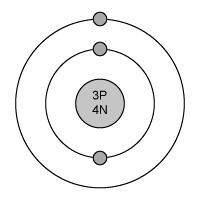

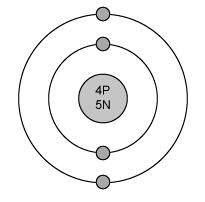
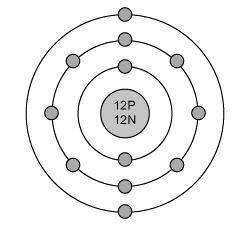
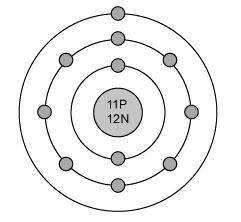

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Questions

Mathematics, 22.11.2021 14:10



English, 22.11.2021 14:10

Mathematics, 22.11.2021 14:10



Mathematics, 22.11.2021 14:10



Arts, 22.11.2021 14:10


History, 22.11.2021 14:10

Mathematics, 22.11.2021 14:10






Computers and Technology, 22.11.2021 14:20



