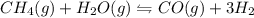Chemistry, 15.12.2019 01:31 montgomerykarloxc24x
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)
what will happen to the equilibrium of this reaction if the pressure is increased?
a. the equilibrium will shift to the left to favor the reverse reaction.
b. the equilibrium will shift to the right to favor the forward reaction.
c. the equilibrium will not be affected by changing the pressure.
d. the equilibrium will not be reestablished after this kind of stress.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
Questions

Mathematics, 17.03.2020 19:54


Biology, 17.03.2020 19:54


Biology, 17.03.2020 19:54

Mathematics, 17.03.2020 19:54




History, 17.03.2020 19:54



English, 17.03.2020 19:54

Mathematics, 17.03.2020 19:54