Chemistry, 04.02.2020 15:50 applereams
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two isotopes, boron-10 and boron-11. determine the percent of each isotope of boron


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

You know the right answer?
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two...
Questions

History, 29.01.2021 18:20

Biology, 29.01.2021 18:20


History, 29.01.2021 18:20

Computers and Technology, 29.01.2021 18:20


Mathematics, 29.01.2021 18:20


Mathematics, 29.01.2021 18:20


Chemistry, 29.01.2021 18:20

Mathematics, 29.01.2021 18:20








Mathematics, 29.01.2021 18:20

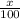
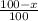
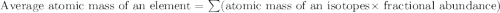
![10.8=\sum[(10)\times \frac{x}{100})+(11)\times \frac{100-x}{100}]]](/tpl/images/0500/8694/6044f.png)