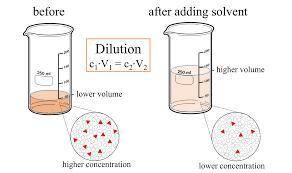
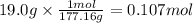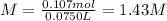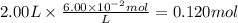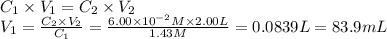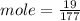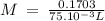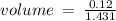On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0g of luminol into a total volume of 75.0ml of h2o.
a)what is the molarity of the stock solution of luminol?
anwer i got: molarity of luminol solution = 1.43m b)before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10−2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces.
i cannot get the correct answer for "c" have tried: 172ml,11.9ml, and 1.19*10^4. the only other possibility that i can come up with is: 83.9ml. would this one be i still completely out to
c)how many moles of luminol are present in 2.00 l of the diluted spray?
anwer i got: moles of luminol = 0.120mol what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?
express your answer in milliliters.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1


Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by...
Questions

Mathematics, 18.09.2019 08:10



History, 18.09.2019 08:10

Health, 18.09.2019 08:10

Mathematics, 18.09.2019 08:10

Mathematics, 18.09.2019 08:10

Mathematics, 18.09.2019 08:10

Health, 18.09.2019 08:10


Spanish, 18.09.2019 08:10




Mathematics, 18.09.2019 08:10

Computers and Technology, 18.09.2019 08:10



Biology, 18.09.2019 08:10

Biology, 18.09.2019 08:10