Which of the following is true in a basic solution?
a. the molarity of hydronium is dou...

Chemistry, 30.10.2019 17:31 msladycie8831
Which of the following is true in a basic solution?
a. the molarity of hydronium is double the molarity of hydroxide.
b. the molarity of hydronium is equal to the molarity of hydroxide.
c. the molarity of hydronium is lower than the molarity of hydroxide.
d. the molarity of hydronium is higher than the molarity of hydroxide.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Questions

Mathematics, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40

Chemistry, 01.03.2021 19:40

Health, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40



Business, 01.03.2021 19:40


Mathematics, 01.03.2021 19:40

History, 01.03.2021 19:40



Mathematics, 01.03.2021 19:40

History, 01.03.2021 19:40

Social Studies, 01.03.2021 19:40

Biology, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40

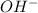 ions as compared to
ions as compared to 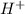 ions and an acidic solution has lesser number of
ions and an acidic solution has lesser number of 

