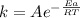Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2

Chemistry, 23.06.2019 15:00
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1
You know the right answer?
If a temperature increase from 12.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is...
Questions


Mathematics, 10.03.2021 01:10


History, 10.03.2021 01:10


Mathematics, 10.03.2021 01:10

Mathematics, 10.03.2021 01:10

Mathematics, 10.03.2021 01:10

Mathematics, 10.03.2021 01:10

Mathematics, 10.03.2021 01:10