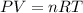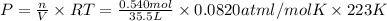Chemistry, 17.10.2019 22:00 samarahbrown6050
What is the pressure of 0.540 mol of an ideal gas at 35.5 l and 223 k? use mc031-1.jpg and mc031-2.jpg.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What is the pressure of 0.540 mol of an ideal gas at 35.5 l and 223 k? use mc031-1.jpg and mc031-2....
Questions


Mathematics, 05.05.2020 12:15



Social Studies, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15



Mathematics, 05.05.2020 12:15




Spanish, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15


Mathematics, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15

Mathematics, 05.05.2020 12:15