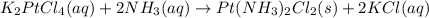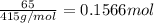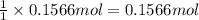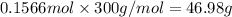Chemistry, 18.11.2019 20:31 shonnybenskin8
The compound cisplatin has been extensively studied as an antitumor agent. it is synthesized by the following, unbalanced reaction. what mass of cisplatin can be made from 65g of k2ptcl4 with sufficient nh3?
k2ptcl4(aq) + nh3(aq) --> pt(nh3)2cl2(s) + kcl(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
The compound cisplatin has been extensively studied as an antitumor agent. it is synthesized by the...
Questions


French, 25.08.2019 12:30

Mathematics, 25.08.2019 12:30



History, 25.08.2019 12:30


Mathematics, 25.08.2019 12:30


Biology, 25.08.2019 12:30



Computers and Technology, 25.08.2019 12:30

Health, 25.08.2019 12:30

Social Studies, 25.08.2019 12:30

Mathematics, 25.08.2019 12:30



Mathematics, 25.08.2019 12:30

Mathematics, 25.08.2019 12:30