
Chemistry, 05.10.2019 17:50 gonzmari457
1. when an acid reacts with a base, what compounds are formed? (1 point) a salt only water only metal oxides only a salt and water2. the formula of the hydrogen ion is often written as (1 point) h2o+ oh+ h+ h4n+ 3. what is an acid according to arrhenius? (1 point) a substance that ionizes to yield protons in aqueous solution a substance that is a hydrogen ion donor a substance that accepts an electron pair a substance that is a hydrogen ion acceptor 4. what is transferred between a conjugate acidbase pair? (1 point) an electron a hydroxide ion a hydrogen ion a hydronium ion 5. which of the following represents a brønstedlowry conjugate acidbase pair? (1 point) so3 2and so2 co3 2and co h3o and h2 nh4 + and nh3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
1. when an acid reacts with a base, what compounds are formed? (1 point) a salt only water only met...
Questions



Mathematics, 23.07.2020 23:01




Mathematics, 23.07.2020 23:01




Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01

Mathematics, 23.07.2020 23:01



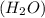 .
.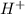 .
.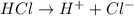
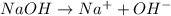
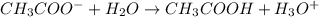
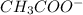 accepts a proton and thus act as a base and the corresponding
accepts a proton and thus act as a base and the corresponding 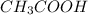 is its conjugate acid.
is its conjugate acid.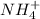 and
and 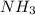
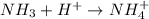
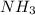 is a acid which accepts proton and thus acts as base to form conjugate acid
is a acid which accepts proton and thus acts as base to form conjugate acid 

