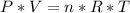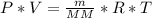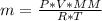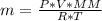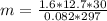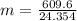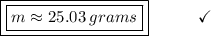Chemistry, 25.08.2019 06:00 princessksh8
How many grams of ethane gas (c2h6) are in 12.7-liter sample at 1.6 atmosphere and 24 degrees celsius? show all work used to solve this problem.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
How many grams of ethane gas (c2h6) are in 12.7-liter sample at 1.6 atmosphere and 24 degrees celsiu...
Questions


Chemistry, 15.04.2021 17:00


Mathematics, 15.04.2021 17:00

History, 15.04.2021 17:00


Chemistry, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Chemistry, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Arts, 15.04.2021 17:00


Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

History, 15.04.2021 17:00

![n = \frac{m}{MM} m (mass) = ? T = 24ºC Celsius to Kelvin TK = TºC + 273 TK = 24 + 273 TK = 297 By the equation of state of the gases or equation of Clapeyron, we have: [tex]P*V = n*R*T](/tpl/images/0195/7138/22057.png)
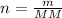 , we can perform the following substitution in the above Clapeyron equation:
, we can perform the following substitution in the above Clapeyron equation: