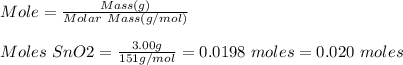Chemistry, 03.02.2020 21:02 alyssamaize
Base your answer to the question on the information below and on your knowledge of chemistry. at 1023 k and 1 atm, a 3.00-gram sample of sno2(s) (gram formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as shown in the balanced equation below. sno2(s) + 2h2(g) → sn(l) + 2h2o(g) show a numerical setup for calculating the number of moles of sno2(s) in the 3.00-gram sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Base your answer to the question on the information below and on your knowledge of chemistry. at 102...
Questions


Mathematics, 04.12.2020 19:40

Biology, 04.12.2020 19:40

History, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40


English, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40



Social Studies, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40



Mathematics, 04.12.2020 19:40



Mathematics, 04.12.2020 19:40