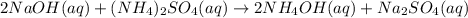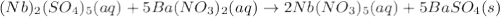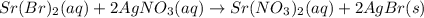Chemistry, 06.10.2019 13:00 genyjoannerubiera
Predict whether each of the following reactions will occur in aqueous solutions. explain your reasoning in each case. note: barium sulfate and silver bromide precipitate in aqueous solutions.
(a) sodium hydroxide + ammonium sulfate → ?
(b) niobium(v) sulfate + barium nitrate → ?
(c) strontium bromide + silver nitrate → ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Predict whether each of the following reactions will occur in aqueous solutions. explain your reason...
Questions



Mathematics, 09.06.2021 20:00



Mathematics, 09.06.2021 20:00

Business, 09.06.2021 20:00



Mathematics, 09.06.2021 20:00

Engineering, 09.06.2021 20:00



Mathematics, 09.06.2021 20:00


Mathematics, 09.06.2021 20:00

Mathematics, 09.06.2021 20:00



Mathematics, 09.06.2021 20:00