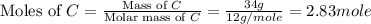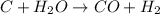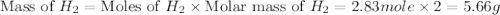Chemistry, 12.01.2020 19:31 ausemkattom3034
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o? the reaction is:
c + h2o → co + h2
the atomic mass of c is 12.01 g/mole. the atomic mass of h2 is 2.016 g/mole. finish the problem by choosing the correct format for dimensional analysis.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2


Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o?...
Questions



Chemistry, 09.02.2021 20:30

Geography, 09.02.2021 20:30

Mathematics, 09.02.2021 20:30


Geography, 09.02.2021 20:30

Mathematics, 09.02.2021 20:30


Social Studies, 09.02.2021 20:30

History, 09.02.2021 20:30

Mathematics, 09.02.2021 20:30



English, 09.02.2021 20:30

Mathematics, 09.02.2021 20:30


Advanced Placement (AP), 09.02.2021 20:30


History, 09.02.2021 20:30

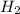 will be, 5.66 grams
will be, 5.66 grams