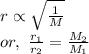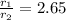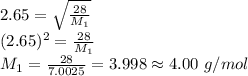Chemistry, 16.10.2019 17:40 cecilysimpson7521
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressure of each gas is known to be 200 torr at 25°c. the gases are allowed to effuse through a pinhole, and it is found that gas a escapes 2.65 times the rate of nitrogen gas. what is the molar mass of gas a?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 15:30
Acontainer holds 6.4 moles of gas. hydrogen gas makes up 25% of the total moles in the container. if the total pressure is 1.24atm. what is the partial pressure of hydrogen
Answers: 3

Chemistry, 23.06.2019 20:00
Using the empirical formula, calculate the formula weight of the compound. use the periodic table to you. type the correct answer in the box. round your answer to three decimal places.
Answers: 1
You know the right answer?
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressu...
Questions


Mathematics, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Law, 20.09.2020 09:01


Social Studies, 20.09.2020 09:01


Chemistry, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01



Business, 20.09.2020 09:01


Business, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01