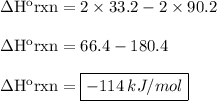Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g)
1/2n2(g)+o2(g)→no2(g), δh∘a=33.2...
1/2n2(g)+o2(g)→no2(g), δh∘a=33.2...
Questions



Advanced Placement (AP), 23.11.2020 23:50

Mathematics, 23.11.2020 23:50

History, 23.11.2020 23:50

Mathematics, 23.11.2020 23:50

Mathematics, 23.11.2020 23:50

Mathematics, 23.11.2020 23:50

History, 23.11.2020 23:50

English, 23.11.2020 23:50

Computers and Technology, 23.11.2020 23:50

Chemistry, 23.11.2020 23:50

English, 23.11.2020 23:50


Mathematics, 23.11.2020 23:50


Mathematics, 23.11.2020 23:50


Mathematics, 23.11.2020 23:50

Health, 23.11.2020 23:50