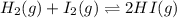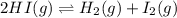Chemistry, 19.11.2019 17:31 mattydoug4818
Two experiments were performed involving the following equilibrium. the temperature was the same in both experiments. h2(g) + i2(g) 2hi(g) in experiment a, 1.0 m i2 and 1.0 m h2 were initially added to a flask and equilibrium was established. in experiment b, 2.0 m hi was initially added to a second flask and equilibrium was established. which of the following statements is always true about the equilibrium concentrations?
a.[h2] equals [hi] in experiment a.
b.[hi] equals 2[h2] in experiment a.
c.[hi] in experiment a equals [hi] in experiment b.
d.[hi] in experiment a equals 1/2[i2] in experiment b.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 13:00
What mass of ca(oh)2 is needed to make 1250ml of a .75m solution?
Answers: 3
You know the right answer?
Two experiments were performed involving the following equilibrium. the temperature was the same in...
Questions

Chemistry, 07.04.2020 09:06

Business, 07.04.2020 09:07

Mathematics, 07.04.2020 09:08

Physics, 07.04.2020 09:08


Mathematics, 07.04.2020 09:08

English, 07.04.2020 09:08

English, 07.04.2020 09:09

Chemistry, 07.04.2020 09:09

Mathematics, 07.04.2020 09:10

Chemistry, 07.04.2020 09:10


Social Studies, 07.04.2020 09:10

English, 07.04.2020 09:11



Engineering, 07.04.2020 09:11


Health, 07.04.2020 09:12

Mathematics, 07.04.2020 09:13