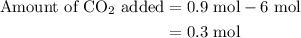Chemistry, 30.01.2020 05:57 Queiao4088
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Spanish, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Biology, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

French, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Health, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

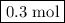 of
of  are added so as to increase the amount of carbon monoxide to 0.3 mol.
are added so as to increase the amount of carbon monoxide to 0.3 mol.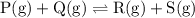
![{\text{K}}=\dfrac{{\left[ {\text{R}} \right]\left[ {\text{S}}\right]}}{{\left[{\text{P}}\right]\left[ {\text{Q}} \right]}}](/tpl/images/0484/7673/9b899.png)

![{\text{K = }}\dfrac{{\left[ {{\text{C}}{{\text{O}}_{\text{2}}}} \right]\left[{{{\text{H}}_{\text{2}}}} \right]}}{{\left[ {{\text{CO}}}\right]\left[{{{\text{H}}_2}{\text{O}}} \right]}}](/tpl/images/0484/7673/6dcad.png) .......(1)
.......(1)![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]](/tpl/images/0484/7673/9014c.png) is the concentration of carbon dioxide.
is the concentration of carbon dioxide.
![\left[{{{\text{H}}_{\text{2}}}}\right]](/tpl/images/0484/7673/340fe.png) is the concentration of hydrogen.
is the concentration of hydrogen.
![\left[{{\text{CO}}}\right]](/tpl/images/0484/7673/d6da7.png) is the concentration of carbon monoxide.
is the concentration of carbon monoxide.
![\left[{{{\text{H}}_2}{\text{O}}}\right]](/tpl/images/0484/7673/62a9e.png) is the concentration of water.
is the concentration of water.
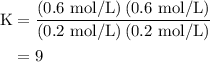
![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]=\dfrac{{{\text{K}}\left( {\left[{{\text{CO}}} \right]\left[{{{\text{H}}_2}{\text{O}}}\right]}\right)}}{{\left[{{{\text{H}}_{\text{2}}}} \right]}}](/tpl/images/0484/7673/f6940.png) ......(2)
......(2)![\begin{aligned}\left[ {{\text{C}}{{\text{O}}_{\text{2}}}}\right]&= \frac{{{\text{9}}\left( {{\text{0}}{\text{.3 mol/L}}}\right)\left({{\text{0}}{\text{.2 mol/L}}}\right)}}{{{\text{0}}{\text{.6 mol/L}}}}\\&= 0.{\text{9 mol/L}}\\\end{aligned}](/tpl/images/0484/7673/95ec6.png)