
Chemistry, 18.09.2019 18:00 shelbycg02
What do colligative properties depend on? a) the identity of the solute b) the number of particles present in the solution c) the quantity of solvent in the solution d) none of these

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
What do colligative properties depend on? a) the identity of the solute b) the number of particles...
Questions

Computers and Technology, 02.12.2020 22:00



Mathematics, 02.12.2020 22:00


Health, 02.12.2020 22:00




Arts, 02.12.2020 22:00

Chemistry, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00

Computers and Technology, 02.12.2020 22:00


Mathematics, 02.12.2020 22:00

English, 02.12.2020 22:00



Business, 02.12.2020 22:00


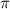 = osmotic pressure
= osmotic pressure


