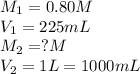Chemistry, 26.10.2019 19:43 skylarrrrr7663
To 225 ml of a 0.80m solution of ki, a student adds enough water to make 1.0l of a more dilute ki solution. what is the molarity of the new solution? a.180m b. 2.8m c. 0.35m d. 0.18m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
To 225 ml of a 0.80m solution of ki, a student adds enough water to make 1.0l of a more dilute ki so...
Questions

Biology, 13.10.2019 05:00


Mathematics, 13.10.2019 05:00

Biology, 13.10.2019 05:00



Mathematics, 13.10.2019 05:00


Biology, 13.10.2019 05:00

History, 13.10.2019 05:00





Mathematics, 13.10.2019 05:01

Arts, 13.10.2019 05:01


Biology, 13.10.2019 05:01


Mathematics, 13.10.2019 05:01


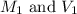 are the molarity and volume of the concentrated solution
are the molarity and volume of the concentrated solution are the molarity and volume of diluted solution
are the molarity and volume of diluted solution