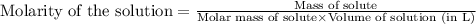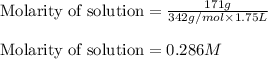Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
There are 342 g of sucrose in 1.00 mol of sucrose. what is the molar concentration (molarity) of a s...
Questions

Mathematics, 04.10.2019 18:00


Biology, 04.10.2019 18:00

History, 04.10.2019 18:00


Mathematics, 04.10.2019 18:00





Mathematics, 04.10.2019 18:00

History, 04.10.2019 18:00



Mathematics, 04.10.2019 18:00



Arts, 04.10.2019 18:00


Biology, 04.10.2019 18:00