
Chemistry, 17.11.2019 02:31 aaronw3743
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were available, how many moles of iron (iii) oxide would be produced?
4 moles
5 moles
6 moles
8 moles

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were...
Questions



Social Studies, 02.08.2021 18:40


English, 02.08.2021 18:40

Social Studies, 02.08.2021 18:40



Mathematics, 02.08.2021 18:40

Health, 02.08.2021 18:40



Biology, 02.08.2021 18:40


History, 02.08.2021 18:40

Mathematics, 02.08.2021 18:40


Mathematics, 02.08.2021 18:40

Mathematics, 02.08.2021 18:40

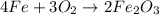
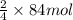 of iron oxide.
of iron oxide.

