Chemistry, 27.09.2019 22:40 baseball1525
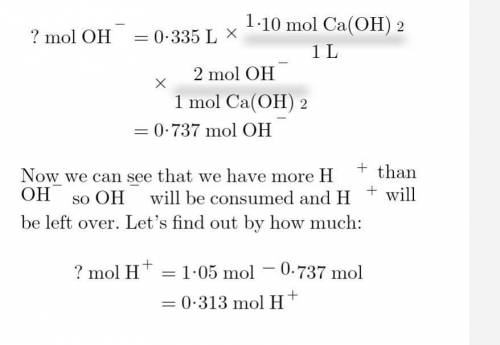
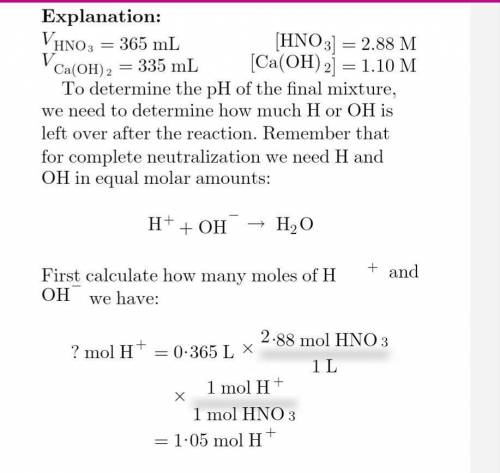
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution....
Questions

History, 31.07.2019 06:00



History, 31.07.2019 06:00


History, 31.07.2019 06:00


Mathematics, 31.07.2019 06:00



History, 31.07.2019 06:00



Geography, 31.07.2019 06:00



English, 31.07.2019 06:00

Mathematics, 31.07.2019 06:00

Physics, 31.07.2019 06:00