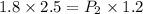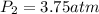Chemistry, 06.10.2019 04:50 drewdean5545
Asample of gas has a pressure of 1.8 atm and a volume of 2.5 l, what would be the pressure of this sample of gas if the volume decreases to 1.2 l? show all of the work used to solve this problem.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Asample of gas has a pressure of 1.8 atm and a volume of 2.5 l, what would be the pressure of this s...
Questions




Medicine, 13.09.2019 23:30


Medicine, 13.09.2019 23:30

Medicine, 13.09.2019 23:30

Biology, 13.09.2019 23:30

Biology, 13.09.2019 23:30


Medicine, 13.09.2019 23:30







Medicine, 13.09.2019 23:30


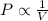 (At constant temperature and number of moles)
(At constant temperature and number of moles)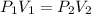
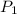 = initial pressure of gas = 1.8 atm
= initial pressure of gas = 1.8 atm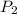 = final pressure of gas = ?
= final pressure of gas = ?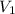 = initial volume of gas = 2.5 L
= initial volume of gas = 2.5 L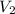 = final volume of gas = 1.2 L
= final volume of gas = 1.2 L