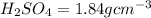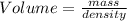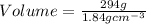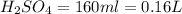2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form liquid sulfuric acid (h2so4) in the unbalanced equation below: so2 + o2 + h2o h2so4 in the laboratory, a chemist carries out this reaction at stp with 67.2 l of sulfur dioxide (so2). how many liters of h2so4 did the chemist produce? 1 mole of any gas = 22.4 l of that same gas at stp • part a: write a balanced equation for the reaction. • part b: calculate the number of liters of h2so4 produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form...
Questions


Mathematics, 05.01.2021 21:40


Social Studies, 05.01.2021 21:40





Mathematics, 05.01.2021 21:40

English, 05.01.2021 21:40






Mathematics, 05.01.2021 21:40



Mathematics, 05.01.2021 21:40

Mathematics, 05.01.2021 21:40

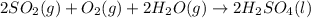
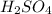 =0.16L
=0.16L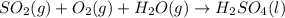
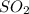 occupies 22.4 L at STP
occupies 22.4 L at STP 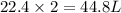 and produce 2 moles of
and produce 2 moles of 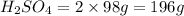
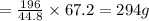 of
of