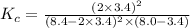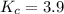Chemistry, 30.01.2020 15:50 sssaaavvvaaagggeee
Suppose 4.2 mol of oxygen and 4.0 mol of no are introduced to an evacuated 0.50-l reaction vessel. at a specific temperature, the equilibrium 2no(g) + o2(g) picture 2no2(g) is reached when [no] = 1.6 m. calculate kc for the reaction at this temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Suppose 4.2 mol of oxygen and 4.0 mol of no are introduced to an evacuated 0.50-l reaction vessel. a...
Questions


Chemistry, 20.08.2019 03:30


Mathematics, 20.08.2019 03:30

Mathematics, 20.08.2019 03:30



Physics, 20.08.2019 03:30

English, 20.08.2019 03:30

History, 20.08.2019 03:30

Biology, 20.08.2019 03:30


Mathematics, 20.08.2019 03:30


History, 20.08.2019 03:30

Health, 20.08.2019 03:30

Social Studies, 20.08.2019 03:30



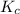 for the reaction is, 3.9
for the reaction is, 3.9 = 4.2 mol
= 4.2 mol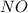 = 4.0 mol
= 4.0 mol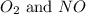 .
.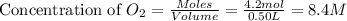
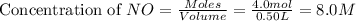
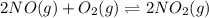
![K_c=\frac{[NO_2]^2}{[NO]^2[O_2]}](/tpl/images/0486/3527/4bf7b.png)
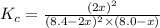 .......(1)
.......(1)