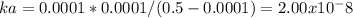12. which compound can act as both a brønstedlowry
acid and a brønstedlowry
base? (1 po...

Chemistry, 04.10.2019 23:00 montecillolinda
12. which compound can act as both a brønstedlowry
acid and a brønstedlowry
base? (1 point)
water
ammonia
sodium hydroxide
hyrdrochloric acid
13. what are the acids in the following equilibrium reaction?
cn– + h2o hcn + oh–
(1 point)
cn–, h2o
h2o, hcn
cn–, oh–
h2o, oh–
14. the products of selfionization
of water are (1 point)
h3o+ and h2o
oh– and oh+
oh+ and h–
oh– and h+
15. which type of solution is one with a ph of 8? (1 point)
acidic
basic
neutral
the type varies, depending on the solution.
16. the acid dissociation constant for an acid dissolved in water is equal to the (1 point)
equilibrium constant
equilibrium constant times the concentration of water
equilibrium constant divided by the concentration of water
equilibrium constant times the equilibrium constant of water
17. a 0.12 m solution of an acid that ionizes only slightly in solution would be termed (1 point)
concentrated and weak
strong and dilute
dilute and weak
concentrated and strong
essay
18. if the solubility of a gas is 7.5 g/l at 404 kpa pressure, what is the solubility of the gas when
the pressure is 202 kpa? show your work.
(3 points)
19. explain on a particle basis how the addition of a solute affects the boiling point, the freezing
point, and the vapor pressure of the solvent.
(6 points)
20.
calculate the hydrogenion
concentration [h+] for the aqueous solution in which [oh–] is 1 x
10–11 mol/l. is this solution acididc, basic, or neutral? show your work.
(3 points)
21. calculate the acid dissociation constant of a weak monoprotic acid if a 0.5m solution of this
acid gives a hydrogenion
concentration of 0.000 1m? show your work.
hint: monoprotic means containing one proton.
(3 points)
22. compare and contrast the properties of acids and bases. include two similarities and two
differences.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
Questions


Biology, 15.08.2019 08:10


Geography, 15.08.2019 08:10


History, 15.08.2019 08:10




Biology, 15.08.2019 08:10

French, 15.08.2019 08:10

History, 15.08.2019 08:10

Mathematics, 15.08.2019 08:10

English, 15.08.2019 08:10


Mathematics, 15.08.2019 08:10


Health, 15.08.2019 08:10


![[H+][OH-]= Kw = 1.0 * 10^-14](/tpl/images/0287/2817/b9e7e.png)
![[H+]= Kw/ [OH-]= 1.0x 10^-14 / 1 x 10^-11 =1 x 10^-3 mol/L pH = - log [H+]= - log 1 x 10^-3 = 3](/tpl/images/0287/2817/63f2b.png)
![ka = [H]^2 / (0.5 - [H+]) ](/tpl/images/0287/2817/777b7.png)