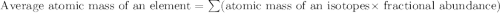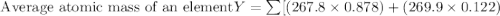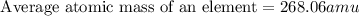Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1

Chemistry, 23.06.2019 16:20
Which of the following is the best name for a compound made from calcium and bromine? (cabr2) calcium bromide calcium dibromide monocalcium dibromide calcium bromine ii
Answers: 1
You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 87.8 percent of the sample is an...
Questions

Mathematics, 19.05.2021 17:50


Chemistry, 19.05.2021 17:50

Health, 19.05.2021 17:50


Biology, 19.05.2021 17:50

Mathematics, 19.05.2021 17:50



English, 19.05.2021 17:50

Mathematics, 19.05.2021 17:50

Mathematics, 19.05.2021 17:50




History, 19.05.2021 17:50



Mathematics, 19.05.2021 17:50