
Chemistry, 01.09.2019 02:30 chriscol4082
Which equivalence factor set should you use to convert 2.68 x 1011 atoms of ag to grams of ag?
(2.68 x 1011 atoms ag/1 mol ag)(1 mol ag/107.88 g ag)
(1 mol ag/2.68 x 1011 atoms ag)(107.88 g ag/1 mol ag)
(1 mol ag/6.02 x 1023 atoms ag)(107.88 g ag/1 mol ag)
(2.68 x 1011 atoms ag/6.02 x 1023 atoms ag)(107.88 g ag)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
Which equivalence factor set should you use to convert 2.68 x 1011 atoms of ag to grams of ag?
Questions


Advanced Placement (AP), 24.03.2021 14:00



History, 24.03.2021 14:00


Physics, 24.03.2021 14:00

Chemistry, 24.03.2021 14:00

English, 24.03.2021 14:00

History, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00

English, 24.03.2021 14:00


English, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00



Mathematics, 24.03.2021 14:00


Mathematics, 24.03.2021 14:00

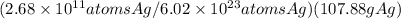
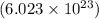 of particles.
of particles.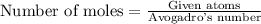
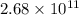
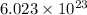
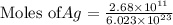
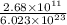 moles of Ag will weigh=
moles of Ag will weigh=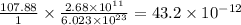 grams.
grams.

