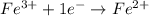When an atom in a reactant gains electrons, what happens to its oxidation number?
its oxidati...

Chemistry, 04.11.2019 18:31 conyabrew82
When an atom in a reactant gains electrons, what happens to its oxidation number?
its oxidation number decreases.
its oxidation number doubles.
its oxidation number increases.
its oxidation number increases by one.
its oxidation number stays the same.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
Questions


Mathematics, 15.07.2020 08:01


Mathematics, 15.07.2020 08:01


Mathematics, 15.07.2020 08:01

English, 15.07.2020 08:01

Mathematics, 15.07.2020 08:01




Mathematics, 15.07.2020 08:01


Chemistry, 15.07.2020 08:01

Biology, 15.07.2020 08:01


History, 15.07.2020 08:01

Mathematics, 15.07.2020 08:01

Mathematics, 15.07.2020 08:01