Chemistry, 13.12.2019 17:31 jladinosolarsee
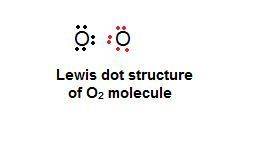
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?
one atom can lose two electrons so that the other atom can gain them and have eight valence electrons.
one atom can lose four electrons to the environment so that a total of eight valence electrons remains.
each atom can share two electrons with the other so that each atom has eight valence electrons.
each atom can lose two electrons so that there is a total of eight valence electrons between the atoms.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3

Chemistry, 23.06.2019 18:30
Match the following items to the correct description. 1. detainment centers for many of hitler's "undesirable" citizens, including those of the jewish race inflation 2. a form of fascist government; probably the most extreme form of tyranny first month of 1933 3. leadership taken and directed by force, often with bloodshed. an oppressive regime tyrannical government 4. financial instability brought on by price increases nazism 5. hitler became the prime minister concentration camps
Answers: 2
You know the right answer?
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?...
Questions

Mathematics, 04.06.2020 20:05


Mathematics, 04.06.2020 20:05

Mathematics, 04.06.2020 20:05


Mathematics, 04.06.2020 20:05


Mathematics, 04.06.2020 20:05

Mathematics, 04.06.2020 20:05

History, 04.06.2020 20:05

English, 04.06.2020 20:05

Mathematics, 04.06.2020 20:05

Mathematics, 04.06.2020 20:05



Mathematics, 04.06.2020 20:05


Mathematics, 04.06.2020 20:05

Chemistry, 04.06.2020 20:05

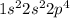
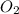 molecule is given in the image attached.
molecule is given in the image attached.