Chemistry, 06.10.2019 05:10 officialgraciela67
When zn(oh)2(s) was added to 1.00 l of a basic solution, 1.09×10−2 mol of the solid dissolved. what is the concentration of oh− in the final solution? \

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
When zn(oh)2(s) was added to 1.00 l of a basic solution, 1.09×10−2 mol of the solid dissolved. what...
Questions



Health, 12.07.2019 04:30


Social Studies, 12.07.2019 04:30


Computers and Technology, 12.07.2019 04:30


Biology, 12.07.2019 04:30

History, 12.07.2019 04:30

History, 12.07.2019 04:30

English, 12.07.2019 04:30



Mathematics, 12.07.2019 04:30



History, 12.07.2019 04:30

Mathematics, 12.07.2019 04:30

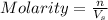
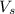 = volume of solution in Liters
= volume of solution in Liters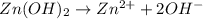
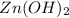 gives = 2 moles of
gives = 2 moles of 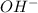
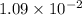 gives =
gives = ![\frac{2}{1}\times 1.09\times 10^{-2}=2.18\times 10^{-2}moles of [tex]OH^-](/tpl/images/0292/2268/6b4cb.png)