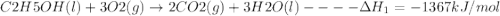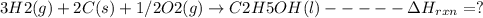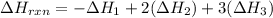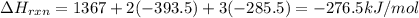Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Using the following thermochemical data, what is the change in enthalpy for the following reaction:...
Questions


Mathematics, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

English, 24.02.2021 01:00


Health, 24.02.2021 01:00

Biology, 24.02.2021 01:00

History, 24.02.2021 01:00

English, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00




English, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

English, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

History, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00