

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
What is the millimolar solubility of oxygen gas, o2, in water at 20 ∘c, if the pressure of oxygen is...
Questions

Mathematics, 21.07.2021 08:20


Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20


Mathematics, 21.07.2021 08:20

Social Studies, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Mathematics, 21.07.2021 08:20

Biology, 21.07.2021 08:20


Mathematics, 21.07.2021 08:20


Mathematics, 21.07.2021 08:20


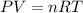
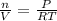

![20^oC=[273+20]=293K](/tpl/images/0485/4904/11ae9.png)




