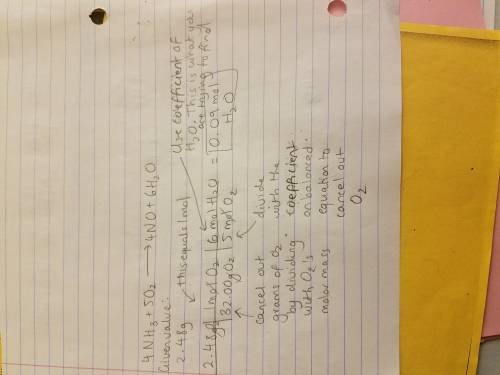Chemistry, 03.02.2020 05:04 Donlito8535
__nh3 + __o2 → __no + __h2o
how many moles of water are produced from
2.48 g of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

You know the right answer?
__nh3 + __o2 → __no + __h2o
how many moles of water are produced from
2.48 g of oxygen?...
how many moles of water are produced from
2.48 g of oxygen?...
Questions

Biology, 10.12.2020 22:30

Law, 10.12.2020 22:30


Mathematics, 10.12.2020 22:30

English, 10.12.2020 22:30


English, 10.12.2020 22:30

Biology, 10.12.2020 22:30

Mathematics, 10.12.2020 22:30


History, 10.12.2020 22:30


Arts, 10.12.2020 22:30

Mathematics, 10.12.2020 22:30


English, 10.12.2020 22:30


Biology, 10.12.2020 22:30