
Chemistry, 14.11.2019 19:31 danessaporter
According to the vsepr theory, what is the shape of a molecule that has a central atom bound to three other atoms with no lone pairs of electrons?
a. tetrahedral
b. bent
c. linear
d. trigonal bipyramidal
e. trigonal planar

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1
You know the right answer?
According to the vsepr theory, what is the shape of a molecule that has a central atom bound to thre...
Questions

Mathematics, 02.07.2020 23:01


History, 02.07.2020 23:01


Chemistry, 02.07.2020 23:01






Mathematics, 02.07.2020 23:01


Mathematics, 02.07.2020 23:01





History, 02.07.2020 23:01

Spanish, 02.07.2020 23:01

![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0374/4553/08b6a.png)
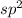 and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.
and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.


