Chemistry, 17.12.2019 09:31 dextor1606
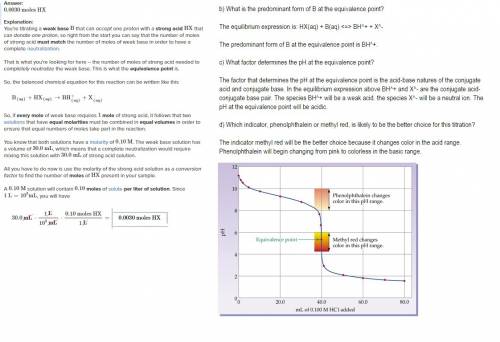
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a 0.10 m solution of a monoprotic strong acid hx. (a) how many moles of hx have been added at the equivalence point? (b) what is the predominant form of b at the equivalence point? (c) what factor determines the ph at the equivalence point? (d) which indicator, phenolphthalein or methyl red, is likely to be the better choice for this titration?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a...
Questions


Mathematics, 15.04.2021 16:20

Mathematics, 15.04.2021 16:20


Mathematics, 15.04.2021 16:20

History, 15.04.2021 16:20

History, 15.04.2021 16:20



History, 15.04.2021 16:20

English, 15.04.2021 16:20


Mathematics, 15.04.2021 16:20




Mathematics, 15.04.2021 16:20

Mathematics, 15.04.2021 16:20