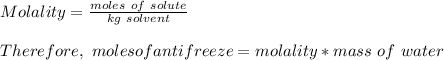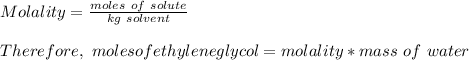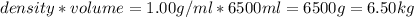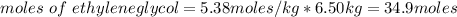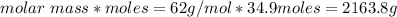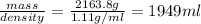Chemistry, 26.09.2019 06:00 inglehailey
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is -10.°c? (the density of ethylene glycol is 1.11 g/ml. assume the density of water at -10.°c is 1.00 g/ml.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator c...
Questions

Mathematics, 11.11.2020 21:40

Chemistry, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40

Biology, 11.11.2020 21:40



History, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40




Mathematics, 11.11.2020 21:40


Advanced Placement (AP), 11.11.2020 21:40


Mathematics, 11.11.2020 21:40

English, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40

English, 11.11.2020 21:40

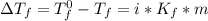
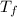 = freezing pt of solution = -10.0 C
= freezing pt of solution = -10.0 C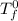 = freezing pt of pure solvent = 0 C
= freezing pt of pure solvent = 0 C![[0-(-10.0)] C= 1*(1.86 C/m) *( m)\\\\m = 5.38](/tpl/images/0263/9231/f27db.png)