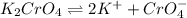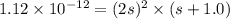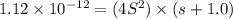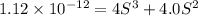Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
Silver chromate is sparingly soluble in aqueous solutions. the ksp of ag2cro4 is 1.12× 10–12. what i...
Questions


Mathematics, 16.10.2019 20:00



Physics, 16.10.2019 20:00


Computers and Technology, 16.10.2019 20:00

English, 16.10.2019 20:00

Mathematics, 16.10.2019 20:00

English, 16.10.2019 20:00

Mathematics, 16.10.2019 20:00

Mathematics, 16.10.2019 20:10






Mathematics, 16.10.2019 20:10

History, 16.10.2019 20:10

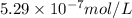
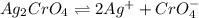
![K_{sp}=[Ag^{+}]^2[CrO_4^{-}]](/tpl/images/0248/2494/607c1.png)
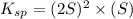
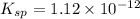
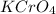 is written as:
is written as: