Idon't have time or the ingredients to do the experiment
materials:
• baking soda
...

Idon't have time or the ingredients to do the experiment
materials:
• baking soda
• vinegar
• measuring cup
• measuring spoons
• 1 latex balloon
• 1 paper or plastic disposable cup
• 1 clear (0.5 l) plastic water bottle or soda bottle (empty and clean)
• 1 marker
• 1 small funnel (optional)
procedures part i:
1. use the paper/plastic cup for this part of the procedure.
2. measure out 118 ml (one half-cup) of vinegar and pour it into the cup.
3. use the marker to draw a line on the cup even with the surface of the vinegar.
4. measure out 1 tablespoon of baking soda.
5. add the baking soda to the vinegar slowly, making sure to not let the bubbles overflow the top of the cup. if bubbles do overflow out of the cup, you will need to perform part i again.
6. record your observations of the reaction, including any volume change (based on the line you drew on the cup).
procedures part ii:
1. use the (0.5 l) plastic bottle and balloon for this part of the procedure.
2. measure out 118 ml (one half-cup) of vinegar and pour carefully into the bottle, using a funnel if you have one.
3. use the marker to draw a line on the bottle even with the surface of the vinegar.
4. measure out 1 tablespoon of baking soda.
5. pour the baking soda carefully into the latex balloon.
6. put the opening of the balloon securely onto the mouth of the bottle, making sure to not spill any baking soda out of the balloon.
7. once the balloon has completely sealed the mouth of the bottle, shake the baking soda down into the vinegar. point the mouth of the bottle away from your face, just in case.
8. gently squeeze the bottle throughout the course of the reaction and record your observations.
9. record all of your observations, in the lab report below, of the reaction, including a comparison of the initial and final volume of liquid in the bottle.
law of conservation of mass activity
lab report
directions: as you complete the conservation of mass activity, record your observations and answers on this lab report. be sure to save the file to be submitted as your assignment for this lesson.
observations: observe all aspects of the reactions carefully and record your observations in the table below.
observations
analysis and conclusion: answer the following questions with complete sentences.
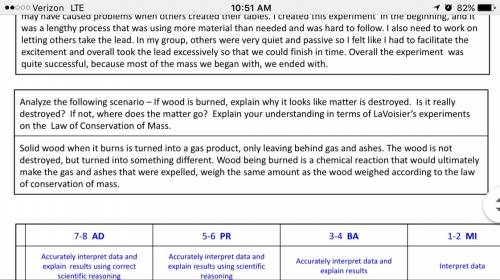
1. if you could measure the mass of the substances inside the cup in part i before and after the reaction, you would see that the mass decreased over the course of the reaction. based on your observations in part i and ii, explain this difference in mass for the unsealed cup.
2. what caused the bubbles in the reaction?
3. if you were to measure the mass before and after the reaction in part ii, you would see that mass was conserved. explain why the law of conservation of mass seemed to be obeyed in part ii but not in part i.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Questions




Biology, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10




Mathematics, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10

Biology, 21.01.2021 19:10