
Chemistry, 08.01.2020 20:31 claudia122752
When sodium is excited in a flame, two ultraviolet spectral lines at lambda - 372.1 nm and lambda = 376.4 nm respectively are emitted . which wavelength represented photons?
a) higher energy?
b) longer wavelengths?
c) higher frequences?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
When sodium is excited in a flame, two ultraviolet spectral lines at lambda - 372.1 nm and lambda =...
Questions









Biology, 25.07.2019 23:30

Mathematics, 25.07.2019 23:30




Mathematics, 25.07.2019 23:40


Biology, 25.07.2019 23:40



Mathematics, 25.07.2019 23:40

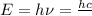 (Planck's equation)
(Planck's equation)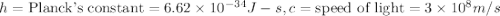
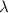 = wavelength of the photon with energy E in meters.
= wavelength of the photon with energy E in meters. = frequency of the photon with energy E in hertz.
= frequency of the photon with energy E in hertz.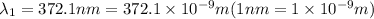
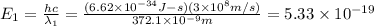 joules
joules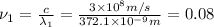 Hertz
Hertz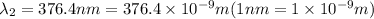
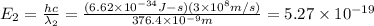 joules
joules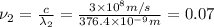 Hertz
Hertz

