
Chemistry, 18.09.2019 17:50 lexybellx3
The temperature of a gas is increased. which statement best explains the effect that this has on the motion of gas particles?
a. the average kinetic energy decreases, and the particles stop colliding.
b. the average kinetic energy increases, and the particles stop colliding.
c. the average kinetic energy decreases, and the particles collide less frequently.
d. the average kinetic energy increases, and the particles collide more frequently.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
The temperature of a gas is increased. which statement best explains the effect that this has on the...
Questions



Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00


Arts, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

Computers and Technology, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

Biology, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00




Chemistry, 15.12.2020 01:00

Chemistry, 15.12.2020 01:00

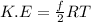
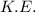 is average kinetic energy,
is average kinetic energy, 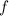 is degree of freedom,
is degree of freedom, 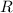 is universal gas constant, and
is universal gas constant, and 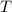 is temperature.
is temperature.


