Acontainer is filled with 4.0 g h2 and 5.0 02. how much water is produced
...

Chemistry, 03.01.2020 23:31 rhiannonwheatcr5743
Acontainer is filled with 4.0 g h2 and 5.0 02. how much water is produced
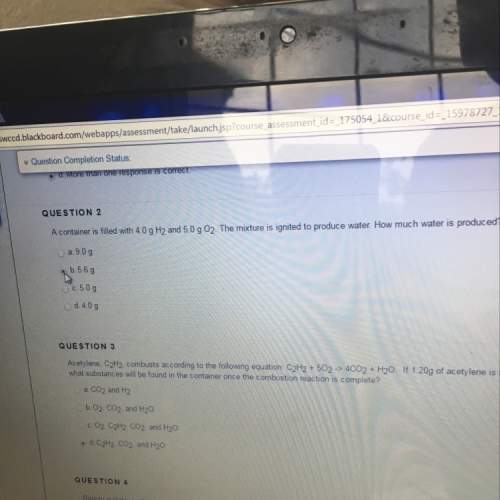

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2
You know the right answer?
Questions

Chemistry, 23.12.2020 23:00


Law, 23.12.2020 23:00



World Languages, 23.12.2020 23:00




Medicine, 23.12.2020 23:00



Mathematics, 23.12.2020 23:00




Chemistry, 23.12.2020 23:00


Social Studies, 23.12.2020 23:00

English, 23.12.2020 23:00



