Chemistry, 19.09.2019 14:50 makennskyee1198
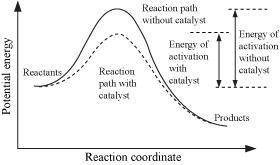
The action of a catalyst can be explained in the following manner: the catalyst lowers the temperature of the reactants. the catalyst takes no part in the reaction but serves as a buffer between reactants and products. the catalyst prevents the reverse reaction. the catalyst makes it possible for the reaction to take place by another path that makes possible reaction at a lower energy.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
The action of a catalyst can be explained in the following manner: the catalyst lowers the temperat...
Questions




Geography, 26.09.2019 16:30

Advanced Placement (AP), 26.09.2019 16:30



History, 26.09.2019 16:30





Mathematics, 26.09.2019 16:30