
Chemistry, 09.11.2019 20:31 elizavlsc4
Consider the rate below
r=k[a][b]^2
which step would quadruple the rate?
doubling the concentration of a
doubling the concentration of b
doubling the concentration of both a and b
doubling the concentration of a but halving the concentration of b

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Consider the rate below
r=k[a][b]^2
which step would quadruple the rate?
doublin...
r=k[a][b]^2
which step would quadruple the rate?
doublin...
Questions

Mathematics, 21.06.2020 04:57


Chemistry, 21.06.2020 04:57



History, 21.06.2020 04:57

Medicine, 21.06.2020 04:57

Mathematics, 21.06.2020 04:57

Mathematics, 21.06.2020 04:57

Mathematics, 21.06.2020 04:57

Mathematics, 21.06.2020 04:57


Mathematics, 21.06.2020 04:57







![R=k[A][B]^2](/tpl/images/0367/2578/b1e88.png)
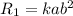
![R_2=k[2a][b]^2=2kab^2=2R_1](/tpl/images/0367/2578/51d77.png)
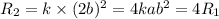
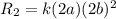
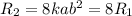
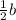
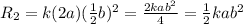
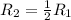
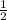 times the rate of initial rate of reaction.Therefore, option D is false.
times the rate of initial rate of reaction.Therefore, option D is false.

