c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1

Chemistry, 02.09.2019 16:00 emily200705
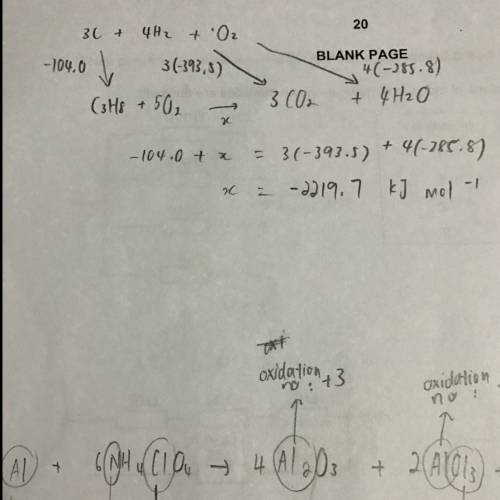
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
h2(g) + o2(g) → h2o(l) ∆h = −285.8 kj mol−1
3c(s) + 4h2(g) → c3h8(g) ∆h = −104.0 kj mol−1
4c(s) + 5h2(g) → c4h10(g) ∆h = −125.2 kj mol−1
the value in kj mol−1
for the enthalpy of combustion of propane is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1


Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
Questions

Physics, 28.10.2019 08:31


Mathematics, 28.10.2019 08:31



Mathematics, 28.10.2019 08:31


English, 28.10.2019 08:31

Mathematics, 28.10.2019 08:31

Social Studies, 28.10.2019 08:31

Biology, 28.10.2019 08:31

Mathematics, 28.10.2019 08:31

History, 28.10.2019 08:31




Mathematics, 28.10.2019 08:31


Mathematics, 28.10.2019 08:31