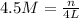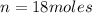Chemistry, 30.09.2019 11:30 jrfranckowiak
How many moles of glucose (c6h12o6) are in 4.0 liters of a 4.5 m c6h12o6 solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
How many moles of glucose (c6h12o6) are in 4.0 liters of a 4.5 m c6h12o6 solution?...
Questions

Mathematics, 17.02.2021 05:00


English, 17.02.2021 05:00

Physics, 17.02.2021 05:00

History, 17.02.2021 05:00

Mathematics, 17.02.2021 05:00

History, 17.02.2021 05:00

Mathematics, 17.02.2021 05:00

History, 17.02.2021 05:00




Mathematics, 17.02.2021 05:00



History, 17.02.2021 05:00

Health, 17.02.2021 05:00

English, 17.02.2021 05:00


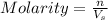
 = volume of solution in L = 4.0 L
= volume of solution in L = 4.0 L