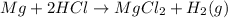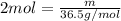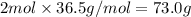Chemistry, 10.10.2019 16:50 shaloveywrighty5965
Curious carl and his lab partner were conducting a variety of experiments to produce gases: hydrogen, oxygen, and carbon dioxide. in one experiment, they added a piece of magnesium ribbon to 10 milliliters of hydrochloric acid. they observed bubbles being produced and did a variety of tests to identify the escaping gas; it proved to be hydrogen. the reaction is represented by the following equation:
mg + 2hcl → mgcl2 + h2(g)
what is the mass, in grams, of two moles of hcl?
a) 18.3 g
b) 36.5 g
c) 72.0 g
d) 73.0 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
Curious carl and his lab partner were conducting a variety of experiments to produce gases: hydroge...
Questions




Mathematics, 09.05.2021 08:10



English, 09.05.2021 08:10

Mathematics, 09.05.2021 08:10

Health, 09.05.2021 08:10




Physics, 09.05.2021 08:10

Biology, 09.05.2021 08:10

Mathematics, 09.05.2021 08:10

Mathematics, 09.05.2021 08:10

Health, 09.05.2021 08:10